A team of French researchers from the Alternative Energies and Atomic Energy Commission has discovered promising evidence of deuterium forming into a metallic state under high pressure. Paul Loubeyre, Florent Occelli, and Paul Dumas describe the process they used to pressurise a deuterium sample and test it for a transition state in their paper published in Physical Review Letters.
According to theory, all elements should transition to a metallic state when subjected to high enough pressure. This is due to the fact that their electrons will eventually become delocalized. However, modeling, let alone demonstrating, such transition points has proven difficult. Early research into hydrogen’s transition state led to theories that it would reach a metallic state when hydrogen molecules completely disassociated.
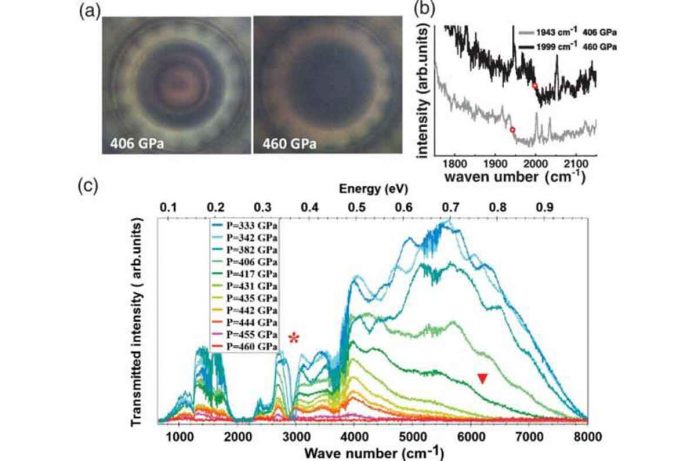
Many attempts were made to determine whether such theories were true, but none were successful.Then, in 2000, a Cornell University team calculated that hydrogen would transition at 410 GPa. In 2020, the current study’s researchers used a diamond anvil cell to compress a sample of hydrogen to 425 GPa and then measured the band gap of the material using synchrotron infrared absorption and Raman spectroscopy. They discovered a sudden drop from 0.6eV to 0.1eV at 80K, indicating that hydrogen is forming into a metallic state as predicted.
Shortly after, physicist Alexander Goncharov proposed that transitions should be easier under conditions where quantum motion allows some atoms to tunnel from one location to another. The researchers reasoned that because deuterium nuclei are heavier than hydrogen, they should be less delocalized than protons and thus require more pressure to transition.To find out, the team reran their 2020 effort, but this time they used deuterium instead of hydrogen. They discovered that the band gap decreased in ways similar to the hydrogen experiment, but at 460 GPa, potentially confirming the theory. The researchers also noted that there was no evidence of molecular disassociation in either experiment.