Scientists have tried to chemically convert carbon dioxide into fuels. But these processes did not work out well in real life. Scientists from MIT have identified the major reason for poor performance in such conversion systems. The reason is a local depletion of the carbon dioxide gas next to the electrodes being used to catalyse the conversion. The problem can be solved by pulsing the current off and on at specific intervals, scientists said. This will allow time for the gas to build back up to the needed levels next to the electrode.
The new finding can spur progress on developing a variety of materials for electrochemical carbon dioxide conversion systems. The study was published in the journal Langmuir.
The research focused on carbon capture and sequestration. In this process the gas is pumped into some kind of deep underground reservoir. It can also be converted to an inert solid such as limestone. The gas can also be converted into other carbon compounds like methane or ethanol.
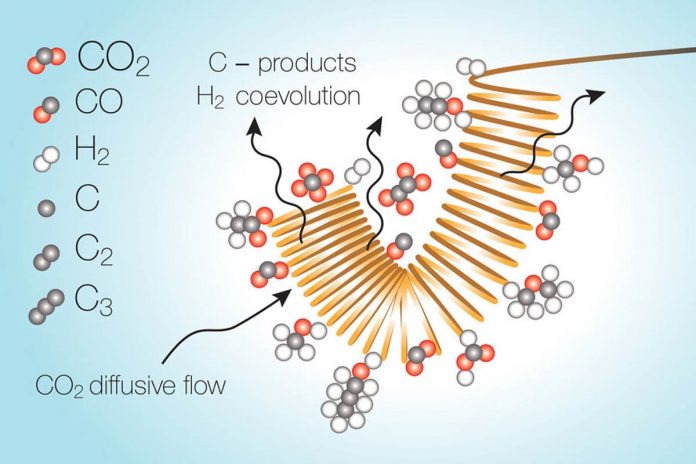
There are many ways such as electrochemical, thermocatalytic, photothermal and photochemical processes for conversion. Scientists have focused on electrochemical approaches to get “higher-C products”. These products contain more carbon atoms and have higher-value fuels because of their energy per weight or volume. The challenge was to curb competing reactions that can take place at the same time. Like the splitting of water molecules into oxygen and hydrogen.
The process take place as a stream of liquid electrolyte with the carbon dioxide dissolved in it passes over a metal catalytic surface that is electrically charged. When carbon dioxide gets converted, it leaves behind a region in the electrolyte stream where it has essentially been used up. The reaction within this depleted zone turns toward water splitting instead. This reaction is unwanted and uses up energy and reduces the overall efficiency of the conversion process.
Pulsed system can counteract this depletion. This system is a cycle of simply turning off the voltage. This system stops the reaction and giving the carbon dioxide time to spread back into the depleted zone and reach usable levels again. Then it resumes the reaction.
The scientists found promising catalyst materials. But they have not run their lab tests long enough to observe these depletion effects. They are trying to scale up their systems. The concentration of carbon dioxide next to the catalyst dictates the products that are made. Depletion can also change the mix of products that are produced and can make the process unreliable.
Scientists developed a simple and reliable way of monitoring the efficiency of the conversion process as it happens. They measured the changing pH levels in the system’s electrolyte.
Scientists used more sophisticated analytical tools to characterize reaction products. They have included gas chromatography for analysis of the gaseous products. They also included nuclear magnetic resonance characterization for the system’s liquid products. Scientists said, their analysis their analysis showed that the simple pH measurement of the electrolyte next to the electrode during operation. This can provide a sufficient measure of the efficiency of the reaction as it progressed.
Easy monitoring the reaction in real-time ultimately lead to a system optimized by machine-learning methods. This controls the production rate of the desired compounds through continuous feedback. Now this process is understood and other approaches to mitigate the carbon dioxide depletion might be developed.